Abstract
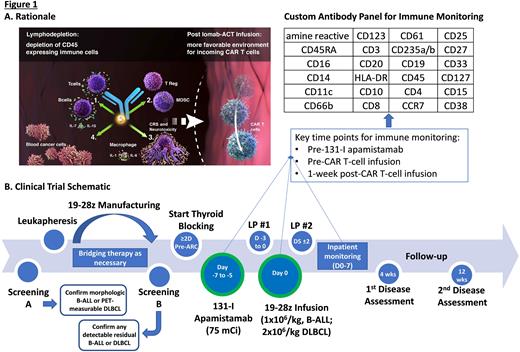
Background: Autologous CD19-targeted chimeric antigen receptor-modified T-cell therapy (CAR-T) is effective over a range of designs for patients (pts) with (w/) relapsed or refractory (R/R) B-cell malignancies including B-cell acute lymphoblastic leukemia (B-ALL) and diffuse large B-cell lymphoma (DLBCL). Within the last year alone, the FDA approved brexu-cel for adults w/ R/R B-ALL and expanded indications for liso-cel and axi-cel to include DLBCL w/ primary refractory disease or early first relapse. While there has been an improvement in the strategies available for prevention and early treatment of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) post-CAR-T, rates of grade ≥3 ICANS exceed 20% even in modern studies utilizing CAR-T w/ a CD28 costimulatory domain (Locke et al., NEJM, 2022; Shah et al., Lancet, 2021) and CRS/ICANS can drive morbidity and prolong inpatient admissions. Anti-cytokine therapies beyond tocilizumab (anti-IL-6R mAb) are being investigated for prevention of CRS/ICANS, including lenzilumab (anti-GM-CSF mAb) and anakinra (IL-1R antagonist). However, as host monocytes appear to be the principal source of cytokines leading to CRS/ICANS (Giavradis et al. and Norelli et al., Nature Medicine, 2018), targeting monocytes w/ conditioning therapy pre-CAR-T may also mitigate toxicity. Low doses of the CD45-targeted antibody radioconjugate (ARC) 131-I apamistamab (Iomab-B; 4-20 mCi) achieve transient lymphodepletion and preclinical studies confirm the potential for low-dose anti-CD45 radioimmunotherapy to achieve profound reductions in B/T-cells, T-regs, splenocytes, and MDSCs w/o targeting BM hematopoietic progenitor cells (Dawicki et al., Oncotarget, 2020). We hypothesized a higher but nonmyeloablative dose of Iomab-B (75 mCi) could serve as lymphodepletion and promote CAR-T expansion while depleting host monocytes. Accordingly, we designed and initiated a pilot study of Iomab-B w/ adoptive cellular therapy (Iomab-ACT; Fig 1A).
Study design and methods: Iomab-ACT is a single-institution pilot study of Iomab-B (w/o chemotherapy) as conditioning prior to 19-28z CAR-T in adults w/ R/R B-ALL or DLBCL (NCT04512716). Pts are eligible for leukapheresis if ≥18 years-old w/ R/R DLBCL (de novo or transformed) following ≥2 chemoimmunotherapy regimens w/ ≥1 FDG-avid measurable lesion or B-ALL following ≥1 line of multi-agent chemotherapy (prior 2nd/3rd gen TKI if Ph+ ALL) w/ ≥5% BM involvement and/or FDG-avid extramedullary disease, ECOG status 0-2 and appropriate organ function. Active or prior CNS disease is not exclusionary. Pts previously treated w/ CD19-targeted CAR T-cell therapy are eligible if disease remains CD19+; pts s/p allogeneic transplant are eligible if not requiring systemic T-cell suppressive therapy. 19-28z CAR-T manufacturing is as previously described (Park et al., NEJM, 2018). Bridging therapy is given at investigator discretion. Thyroid blocking is started ≥48h pre-ARC. Iomab-B (75 mCi) is administered 5-7d (goal 6d) pre-CAR-T infusion. 19-28z CAR-T is administered as a single infusion (1x106/kg, B-ALL pts; 2x106/kg, DLBCL pts; Fig 1B).
The trial uses a 3+3 design in a single cohort; 3 pts have been treated. Unexpected toxicity (given low dose of ARC) observed in 1 pt included severe trilineage cytopenias lasting >8 wks (requiring RBC/PLT transfusion support, G-CSF, romiplostim) w/o marrow hypoplasia and w/o other apparent neoplastic or drug-induced etiology; this met criteria for dose-limiting toxicity and we will monitor in the next 3 pts. Notably, 3 pts underwent leukapheresis, but were not treated (2 died w/ refractory ALL pre-ARC/CAR-T and 1 w/ DLBCL achieved CR w/ bridging), highlighting challenges w/ conduct of autologous CAR T-cell trials in this population.
The primary objective is to determine safety/tolerability of Iomab-B (75 mCi) prior to 19-28z CAR-T. Secondary objectives include determining incidence/severity of ICANS/CRS, anti-tumor efficacy, and CAR-T expansion/persistence by flow cytometry/qPCR. Key exploratory objectives include describing changes in circulating immune cells following ARC and 19-28z CAR T-cells w/spectral cytometry using a custom antibody panel (Fig 1B) and cytokine levels in cerebrospinal fluid. We hope to generate preliminary data to guide further study of CD45-targeted ARCs prior to CAR-T and other forms of adoptive cellular therapy.
Disclosures
Geyer:Sanofi: Consultancy, Research Funding; Amgen: Research Funding; Allogene: Consultancy; Actinium Pharmaceuticals, Inc: Research Funding. Spross:Actinium Pharmaceuticals, Inc: Current Employment. Mark:Actinium Pharmaceuticals, Inc: Current Employment. Desai:Actinium Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Palomba:Ceramedix: Consultancy, Honoraria; BeiGene: Consultancy; Kite, a Gilead Company: Consultancy; Novartis: Consultancy; PCYC: Consultancy; Notch: Current equity holder in private company, Honoraria; Seres: Current equity holder in private company, Honoraria, Patents & Royalties, Research Funding; Lygenesis: Honoraria; Magenta: Honoraria; Nektar: Honoraria; Pluto: Honoraria; Priothera: Honoraria; Rheos: Honoraria; WindMIL: Honoraria; Juno: Patents & Royalties; Wolters Kluwer: Patents & Royalties. Pandit-Taskar:Bristol Myers Squibb: Research Funding; Bayer: Research Funding; Clarity Pharma: Research Funding; Illumina: Consultancy; ImaginAb: Consultancy, Honoraria, Research Funding; Ymabs: Research Funding; Progenics: Consultancy, Honoraria; Medimmune/Astrazeneca: Consultancy, Honoraria; Actinium Pharmaceuticals, Inc: Consultancy, Honoraria; Janssen: Research Funding; Regeneron: Research Funding.
OffLabel Disclosure:
131-I apamistamab and 19-28z CAR T-cells are investigational agents in treatment of ALL and DLBCL
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal